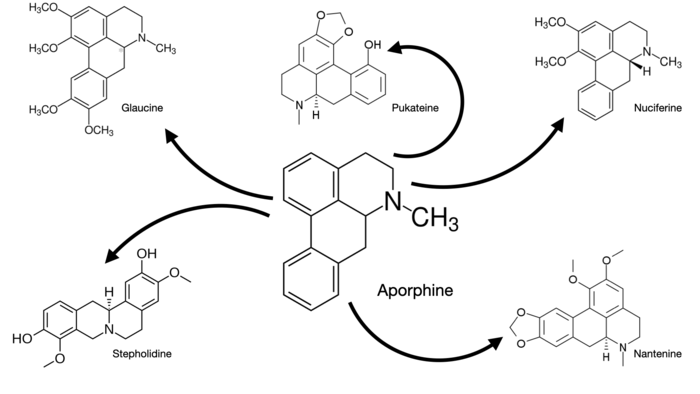
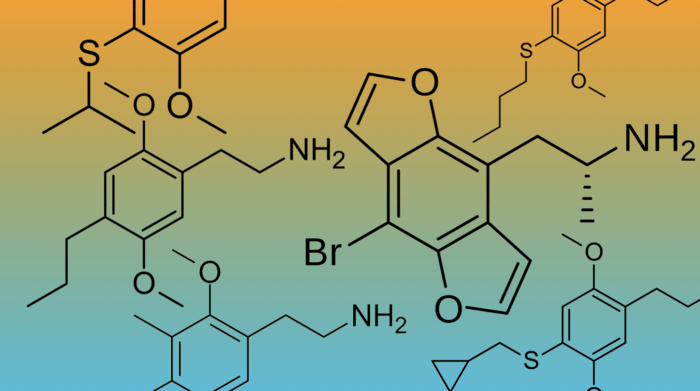
Isoquinolines: structural analogues of the mysterious third family
Isoquinolines are a rather large naturally-occurring family of benzopyridines which find medical applications in everything from antihypertensives to anti-retrovirals to anesthetics. But they have an interesting hidden side which is to date pretty unexplored.
Those familiar with Alex Shulgin’s work will no doubt remember making their way through PIHKAL, first through the autobiographical chapters and then the synthesis portion where phenethylamines (a family which includes 2C-B, 2C-C, MDMA, and mescaline) are described in impressive detail. Some have also picked up TIHKAL, the continuation of the journey through the space of hallucinogenic compounds, this time covering tryptamines (psilocin, bufotenine, DMT, and LSD) with the same basic book structure.
Not including the various compiled studies and reports that Shulgin wrote over the course of his career, there is a third major...